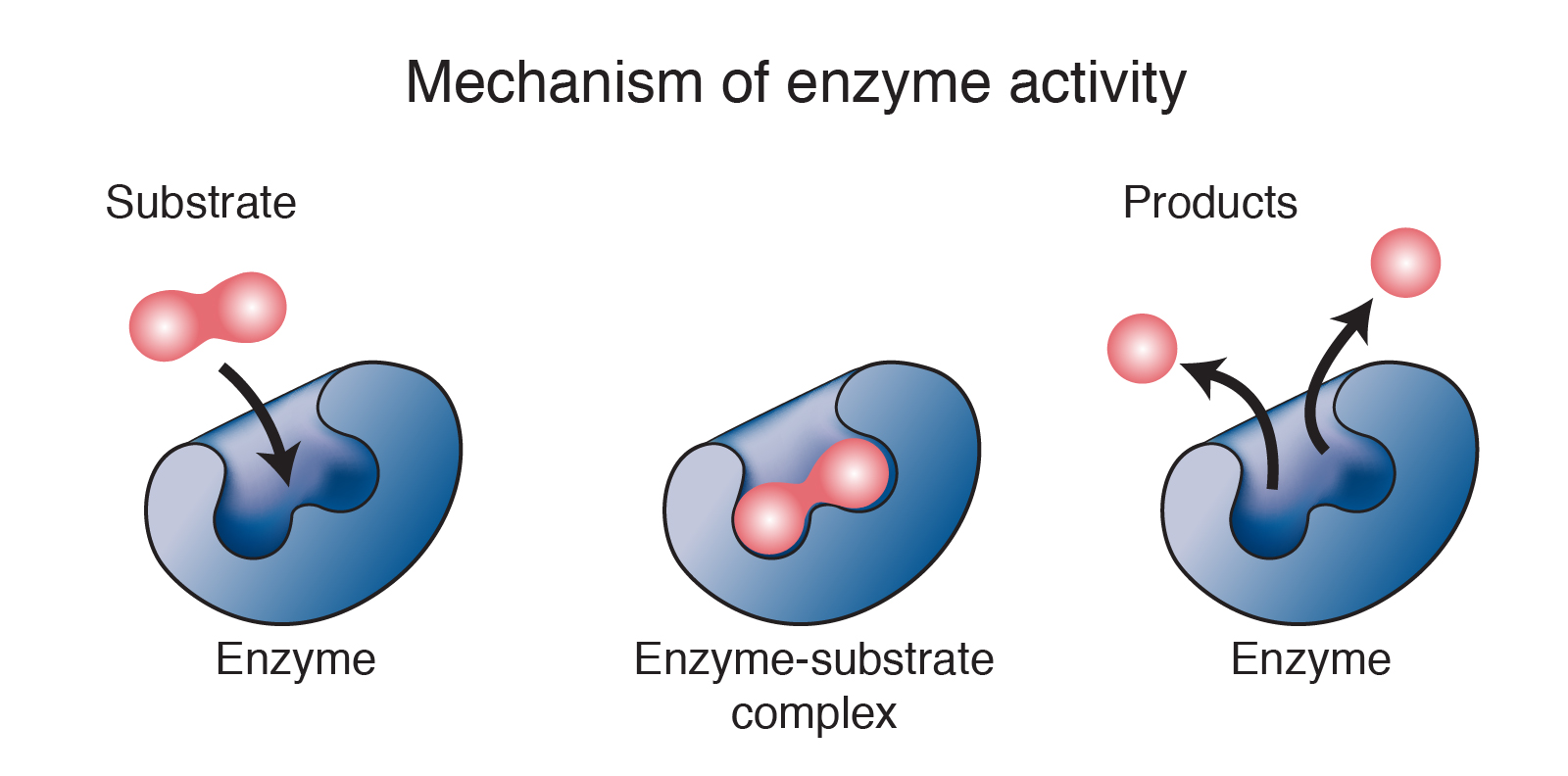
Enzymes Lower Energy Barrier . Enzymes can lower activation energy by binding substrates individually and bringing them together under optimal conditions to react. One of the ways the activation energy is lowered is. Enzymes can lower the activation energy of a chemical reaction in three ways. Enzymes lower the activation energy necessary to transform a reactant into a product. On the left is a reaction that is not catalyzed by an enzyme (red), and on the right is one that is. Consider a reaction in which substrates a and b. The energy required to reach the transition state (the activation energy) constitutes a barrier to the progress of the reaction, limiting the rate of the reaction. Enzymes speed up reactions by lowering the activation energy barrier enzymes are biological catalysts, and therefore not consumed or altered by the reactions they catalyze.
from www.genome.gov
Consider a reaction in which substrates a and b. The energy required to reach the transition state (the activation energy) constitutes a barrier to the progress of the reaction, limiting the rate of the reaction. One of the ways the activation energy is lowered is. On the left is a reaction that is not catalyzed by an enzyme (red), and on the right is one that is. Enzymes can lower activation energy by binding substrates individually and bringing them together under optimal conditions to react. Enzymes speed up reactions by lowering the activation energy barrier enzymes are biological catalysts, and therefore not consumed or altered by the reactions they catalyze. Enzymes can lower the activation energy of a chemical reaction in three ways. Enzymes lower the activation energy necessary to transform a reactant into a product.
Enzyme
Enzymes Lower Energy Barrier Enzymes can lower the activation energy of a chemical reaction in three ways. Enzymes can lower the activation energy of a chemical reaction in three ways. One of the ways the activation energy is lowered is. The energy required to reach the transition state (the activation energy) constitutes a barrier to the progress of the reaction, limiting the rate of the reaction. On the left is a reaction that is not catalyzed by an enzyme (red), and on the right is one that is. Consider a reaction in which substrates a and b. Enzymes speed up reactions by lowering the activation energy barrier enzymes are biological catalysts, and therefore not consumed or altered by the reactions they catalyze. Enzymes can lower activation energy by binding substrates individually and bringing them together under optimal conditions to react. Enzymes lower the activation energy necessary to transform a reactant into a product.
From www.slideserve.com
PPT Enzyme Rate Enhancement PowerPoint Presentation, free download Enzymes Lower Energy Barrier Consider a reaction in which substrates a and b. Enzymes speed up reactions by lowering the activation energy barrier enzymes are biological catalysts, and therefore not consumed or altered by the reactions they catalyze. Enzymes can lower activation energy by binding substrates individually and bringing them together under optimal conditions to react. On the left is a reaction that is. Enzymes Lower Energy Barrier.
From chrismasterjohnphd.com
2. Activation Energy and Enzymes Chris Masterjohn, PhD Enzymes Lower Energy Barrier On the left is a reaction that is not catalyzed by an enzyme (red), and on the right is one that is. Enzymes lower the activation energy necessary to transform a reactant into a product. Enzymes can lower the activation energy of a chemical reaction in three ways. Enzymes speed up reactions by lowering the activation energy barrier enzymes are. Enzymes Lower Energy Barrier.
From www.slideserve.com
PPT ENZYMES PowerPoint Presentation, free download ID6886656 Enzymes Lower Energy Barrier One of the ways the activation energy is lowered is. Enzymes speed up reactions by lowering the activation energy barrier enzymes are biological catalysts, and therefore not consumed or altered by the reactions they catalyze. Enzymes can lower activation energy by binding substrates individually and bringing them together under optimal conditions to react. Consider a reaction in which substrates a. Enzymes Lower Energy Barrier.
From www.slideserve.com
PPT Enzymes PowerPoint Presentation, free download ID2265387 Enzymes Lower Energy Barrier Enzymes can lower the activation energy of a chemical reaction in three ways. The energy required to reach the transition state (the activation energy) constitutes a barrier to the progress of the reaction, limiting the rate of the reaction. Enzymes lower the activation energy necessary to transform a reactant into a product. Enzymes can lower activation energy by binding substrates. Enzymes Lower Energy Barrier.
From studylib.net
Enzymes Lower Activation Energy Enzymes Lower Energy Barrier The energy required to reach the transition state (the activation energy) constitutes a barrier to the progress of the reaction, limiting the rate of the reaction. Consider a reaction in which substrates a and b. One of the ways the activation energy is lowered is. Enzymes speed up reactions by lowering the activation energy barrier enzymes are biological catalysts, and. Enzymes Lower Energy Barrier.
From www.expii.com
Rate of Reaction (Enzymes) — Role & Importance Expii Enzymes Lower Energy Barrier One of the ways the activation energy is lowered is. On the left is a reaction that is not catalyzed by an enzyme (red), and on the right is one that is. Enzymes speed up reactions by lowering the activation energy barrier enzymes are biological catalysts, and therefore not consumed or altered by the reactions they catalyze. Enzymes can lower. Enzymes Lower Energy Barrier.
From www.youtube.com
Enzymes and Activation Energy YouTube Enzymes Lower Energy Barrier Enzymes can lower activation energy by binding substrates individually and bringing them together under optimal conditions to react. One of the ways the activation energy is lowered is. Enzymes speed up reactions by lowering the activation energy barrier enzymes are biological catalysts, and therefore not consumed or altered by the reactions they catalyze. Enzymes lower the activation energy necessary to. Enzymes Lower Energy Barrier.
From www.worthington-biochem.com
Enzyme Energy Levels Worthington Biochemical Enzymes Lower Energy Barrier Enzymes speed up reactions by lowering the activation energy barrier enzymes are biological catalysts, and therefore not consumed or altered by the reactions they catalyze. Consider a reaction in which substrates a and b. Enzymes can lower the activation energy of a chemical reaction in three ways. On the left is a reaction that is not catalyzed by an enzyme. Enzymes Lower Energy Barrier.
From www.studeersnel.nl
Voeding en energie les 4 Enzymes speed up metabolic reactions by Enzymes Lower Energy Barrier Enzymes speed up reactions by lowering the activation energy barrier enzymes are biological catalysts, and therefore not consumed or altered by the reactions they catalyze. Enzymes lower the activation energy necessary to transform a reactant into a product. One of the ways the activation energy is lowered is. On the left is a reaction that is not catalyzed by an. Enzymes Lower Energy Barrier.
From dokumen.tips
(PPT) Enzymes speed up metabolic reactions by lowering energy barriers Enzymes Lower Energy Barrier The energy required to reach the transition state (the activation energy) constitutes a barrier to the progress of the reaction, limiting the rate of the reaction. One of the ways the activation energy is lowered is. Enzymes lower the activation energy necessary to transform a reactant into a product. Enzymes speed up reactions by lowering the activation energy barrier enzymes. Enzymes Lower Energy Barrier.
From www.slideserve.com
PPT 6.2 Enzymes and Chemical Reactions pages 156160 PowerPoint Enzymes Lower Energy Barrier The energy required to reach the transition state (the activation energy) constitutes a barrier to the progress of the reaction, limiting the rate of the reaction. Enzymes speed up reactions by lowering the activation energy barrier enzymes are biological catalysts, and therefore not consumed or altered by the reactions they catalyze. On the left is a reaction that is not. Enzymes Lower Energy Barrier.
From www.slideserve.com
PPT CHAPTER 3 PowerPoint Presentation, free download ID1361511 Enzymes Lower Energy Barrier On the left is a reaction that is not catalyzed by an enzyme (red), and on the right is one that is. Enzymes speed up reactions by lowering the activation energy barrier enzymes are biological catalysts, and therefore not consumed or altered by the reactions they catalyze. Enzymes lower the activation energy necessary to transform a reactant into a product.. Enzymes Lower Energy Barrier.
From www.genome.gov
Enzyme Enzymes Lower Energy Barrier On the left is a reaction that is not catalyzed by an enzyme (red), and on the right is one that is. Enzymes lower the activation energy necessary to transform a reactant into a product. One of the ways the activation energy is lowered is. Enzymes speed up reactions by lowering the activation energy barrier enzymes are biological catalysts, and. Enzymes Lower Energy Barrier.
From slideplayer.com
Energy, Enzymes, and Metabolism ppt download Enzymes Lower Energy Barrier On the left is a reaction that is not catalyzed by an enzyme (red), and on the right is one that is. Enzymes lower the activation energy necessary to transform a reactant into a product. Enzymes speed up reactions by lowering the activation energy barrier enzymes are biological catalysts, and therefore not consumed or altered by the reactions they catalyze.. Enzymes Lower Energy Barrier.
From www.slideserve.com
PPT Designing tomorrow’s drugs PowerPoint Presentation, free download Enzymes Lower Energy Barrier One of the ways the activation energy is lowered is. The energy required to reach the transition state (the activation energy) constitutes a barrier to the progress of the reaction, limiting the rate of the reaction. Enzymes can lower activation energy by binding substrates individually and bringing them together under optimal conditions to react. Enzymes can lower the activation energy. Enzymes Lower Energy Barrier.
From study.com
Function of Enzymes Substrate, Active Site & Activation Energy Video Enzymes Lower Energy Barrier Enzymes lower the activation energy necessary to transform a reactant into a product. Enzymes can lower the activation energy of a chemical reaction in three ways. Consider a reaction in which substrates a and b. On the left is a reaction that is not catalyzed by an enzyme (red), and on the right is one that is. Enzymes speed up. Enzymes Lower Energy Barrier.
From www.slideserve.com
PPT Endergonic and Exergonic Reactions PowerPoint Presentation ID Enzymes Lower Energy Barrier Consider a reaction in which substrates a and b. On the left is a reaction that is not catalyzed by an enzyme (red), and on the right is one that is. Enzymes can lower the activation energy of a chemical reaction in three ways. Enzymes speed up reactions by lowering the activation energy barrier enzymes are biological catalysts, and therefore. Enzymes Lower Energy Barrier.
From www.youtube.com
Enzyme Basics & How Do They Lower Activation Energy? YouTube Enzymes Lower Energy Barrier The energy required to reach the transition state (the activation energy) constitutes a barrier to the progress of the reaction, limiting the rate of the reaction. Consider a reaction in which substrates a and b. Enzymes lower the activation energy necessary to transform a reactant into a product. Enzymes speed up reactions by lowering the activation energy barrier enzymes are. Enzymes Lower Energy Barrier.